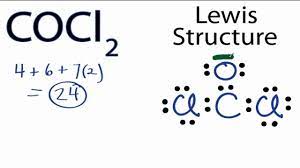
Hey, chemistry enthusiast! Ever found yourself puzzled by those quirky diagrams with dots and lines, symbolizing atoms and their bonds? Well, we’re diving deep into one of those today. Stick around, and by the end, you’ll be a pro at drawing the Lewis Structure of COCl2.
Introduction to Lewis Structures
What is a Lewis Structure?
At its core, a Lewis structure visually represents the molecular arrangement and the electrons around atoms. Think of it as a snapshot of how atoms bond and share electrons. Cool, right?
Importance of Lewis Structures in Chemistry
Not only are these diagrams fun (for some of us!) to draw, but they’re essential. They show how atoms bond, hint at the molecule’s shape, and even give a clue about its reactivity.
Basics Before Drawing
Understanding Atomic Valence Electrons
Before sketching anything, let’s chat about valence electrons. These are the outermost electrons and play a pivotal role in bonding. Picture them as friendly neighbors wanting to shake hands, or in this case, bond with other atoms.
Importance of Electronegativity
Electronegativity is a measure of how much an atom loves electrons. More the love, tighter the hold! This concept will guide you when guessing which atoms might share or hog electrons.
Octet Rule in Brief
Remember this rule: most atoms like having 8 electrons in their outer shell. It’s like the golden number for stability.
Step-by-Step Guide to Draw the Lewis Structure for COCl2
Counting the Total Valence Electrons
First thing’s first, tally up those electrons! Carbon has 4, oxygen has 6, and chlorine has 7, but we have two of them, so that’s 14. All added up, we get 24 electrons to play with.
Determining the Central Atom
Carbon is like that popular kid in school; it’s almost always in the center. So, for COCl2, Carbon is our star player.
Placing the Bonds
Each bond represents 2 electrons. So, connecting C-O and C-Cl twice (because there are two Cl atoms) uses up 8 electrons. 16 more to go!
Distributing the Remaining Electrons
Spread out the leftover electrons to satisfy the octet rule. Oxygen and Chlorines will hog most of these because of their high electronegativity.
Checking the Octet Rule
Finally, do a quick check. Every atom, except hydrogen, should follow this rule. If not, you might need some double or triple bonds. But for COCl2, we’re all set!
Possible Mistakes and Troubleshooting
Common Misconceptions
Don’t forget: not every atom will always follow the octet rule. And sometimes, atoms can share more electrons through double or triple bonds.
Double and Triple Bonds
If you’re scratching your head wondering why some atoms aren’t happy with a single bond, remember that sharing is caring. Some atoms share more electrons to reach that golden number.
Conclusion and Key Takeaways
Drawing the Lewis Structure of COCl2 isn’t so daunting, right? With a sprinkle of patience and a dash of practice, you’ll sketch these structures in no time. Remember the key steps: count, place, distribute, and check!
FAQs
- Why is the octet rule important?
- The octet rule is like an atom’s rule-of-thumb for stability. Most atoms crave that balance, which dictates how they bond with others.
- Can an atom have more than 8 electrons?
- Yes! Some heavier elements can exceed the octet rule by using their d-orbitals.
- Why is carbon often the central atom?
- Carbon’s ability to form four bonds makes it an ideal central player in many molecules.
- What’s the significance of double or triple bonds?
- They allow atoms to share more electrons, which often helps satisfy the octet rule.
- How often do I need to consider electronegativity when drawing Lewis structures?
- Electronegativity will often guide electron distribution, so it’s good practice to keep it in mind!

